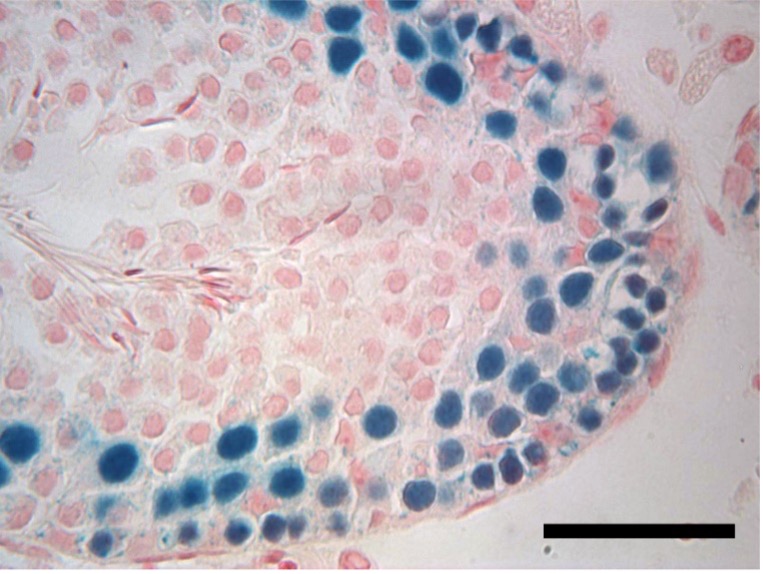
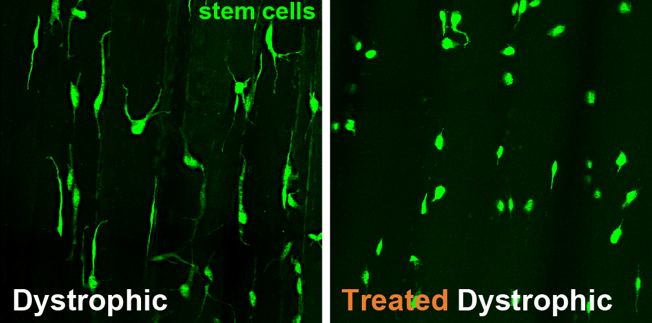
Unlike women, who are born with all the eggs they’ll ever have, men can continue to produce sperm throughout their adult lives. To do so, they require a constant renewal of spermatogonial stem cells, which give rise to sperm.
This reinvigoration of stem cells depends on a newly characterized stem cell self-renewal factor called DOT1L, according to research by Jeremy Wang of the University of Pennsylvania School of Veterinary Medicine and colleagues. When mice lack DOT1L, the team showed, they fail to maintain spermatogonial stem cells, and thus, lack the ability to continuously produce sperm.
Scientists have discovered only a handful of such stem cell renewal factors, so the find, published in the journal Genes & Development, adds another entity to a rarified group.
“This novel factor was only able to be identified by finding this unusual genetic phenotype: the fact that mice lacking DOT1L were not able to continue to produce sperm,” says Wang, the Ralph L. Brinster President’s Distinguished Professor at Penn Vet and a corresponding author on the paper. “Identifying this essential factor not only helps us understand the biology of adult germline stem cells, but could also allow scientists to one day reprogram somatic cells, like skin cells, to become germline stem cells. That is the next frontier for fertility treatment.”
Read more about this research in Penn Today.