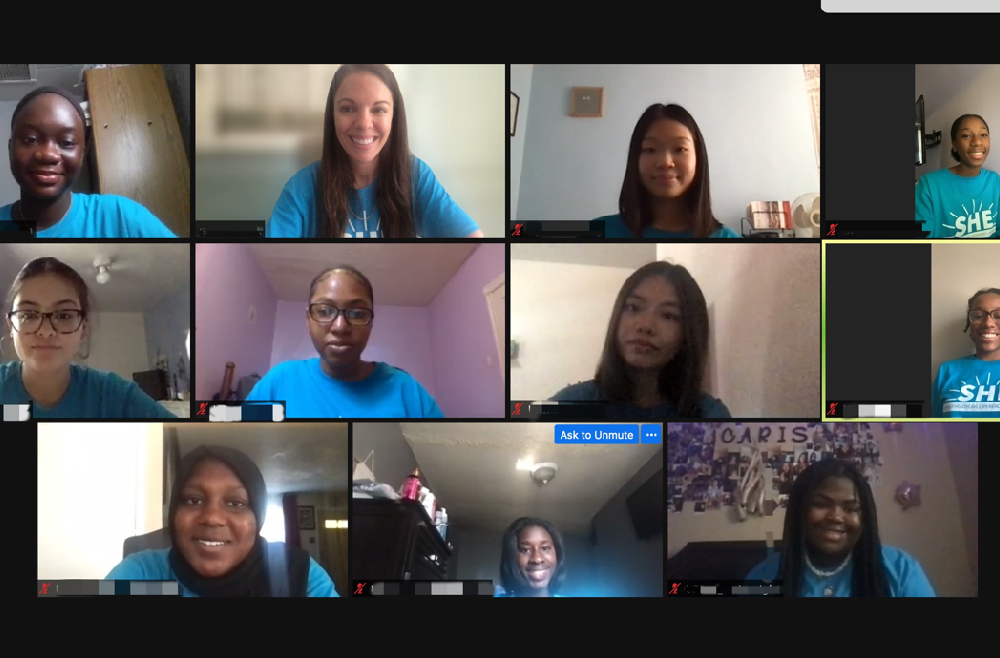
For two weeks in July, a computer screen gave 18-year-old Bintou Samassa glimpses of what she wants her future to look like.
She watched and listened to Yehoda M. Martei, MD, an assistant professor of Hematology-Oncology in the Perelman School of Medicine at the University of Pennsylvania, talk about health disparities one day, while Armenta Washington, a senior research coordinator at the Abramson Cancer Center (ACC), discussed community engagement on another. Before that, Donita C. Brady, PhD, a Presidential professor of Cancer Biology at Penn, had walked through what it’s like to work in a lab.
Enrolling in the ACC’s Summer Health Experience, or SHE, program that introduces young women to careers in cancer, had placed her virtually in front of a group she doesn’t often see: Black women in medicine.
“The goal is simple: to engage and support underserved students,” said Jamie Shuda, EdD, the director of outreach, education, and research at the Perelman School of Medicine, and one of the leaders of the SHE Program at Penn. “An impactful outcome of this virtual program has been the exposure of SHE participants to each other. These students were eager to learn about and explore cancer care in geographic locations outside of their own. Their own scientific literacy has grown alongside the opportunity for them to find STEM mentors and a new peer network.”
Read more about the ACC Summer Health Experience in Penn Medicine News.