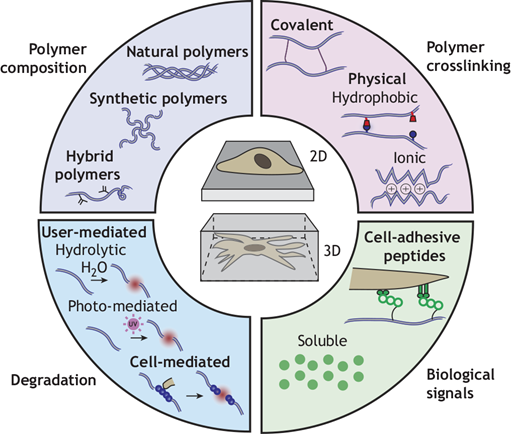
A team of researchers including Robert Mauck, PhD, the Mary Black Ralston Professor of Orthopaedic Surgery and Professor of Bioengineering at the University of Pennsylvania recently covered how engineered hydrogels can serve as in vitro culture platforms to present such signals in a controlled manner and include examples of how they have been used to advance our understanding of developmental biology. You can read more about this research in Development.
Dr. Mauck also co-directs the Program in Musculoskeletal Regeneration at the IRM. Specific information about current work in the Mauck Lab can be found here.