The rate of survival for childhood cancers has increased dramatically in the last several decades, but a serious side effect of treatment is diminished fertility later in life. A potential treatment for boys facing cancer treatment would be to harvest, freeze, and, after the cancer is cured, reimplant their testicular tissue, which contains stem cells that could give rise to sperm.
What happens to that tissue, however, when subjected to the long-term freezing that could be necessary, has remained unclear.
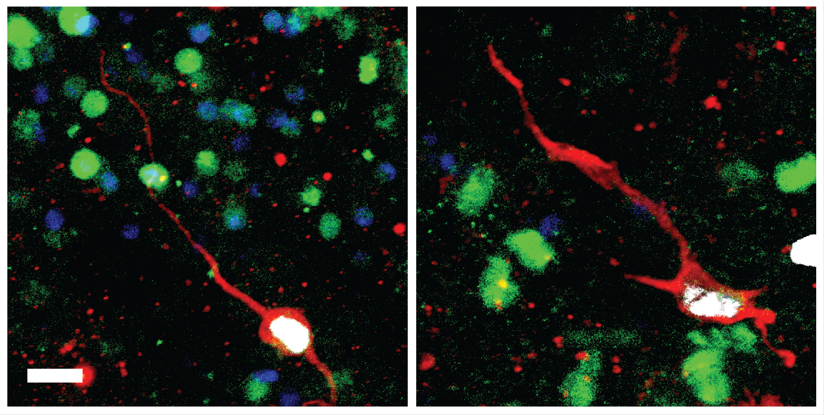
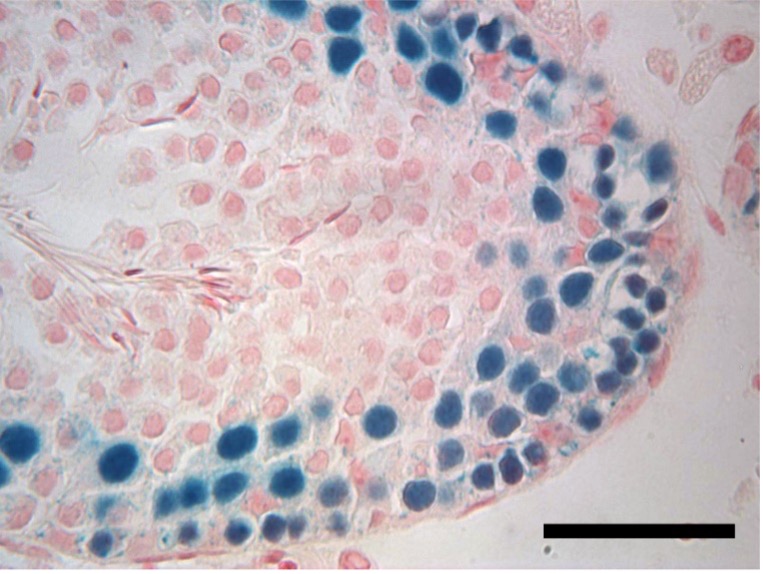
A new study in rats led by University of Pennsylvania School of Veterinary Medicine researchers has shown that male testis tissue that is cryopreserved can be reimplanted after more than 20 years and will go on to make viable sperm. The work, led by senior research investigator Eoin C. Whelan, was published in PLOS Biology.
While the long-frozen testicular tissue could produce sperm, the team found that the long delay did come with a cost in reduced sperm production compared to tissue that is only briefly frozen. The results may have important implications for treatment of prepubertal boys with cancer, for whom chemotherapy may be preceded by harvesting and freezing of testicular tissue for eventual reimplantation.
“The glass-half-empty way of looking at this is that stem cells do seem to be compromised in their ability to regenerate sperm after a long freezing time,” Whelan says. “But the good news is that sperm can be produced, and they seem to be transcriptionally normal when we examined their RNA.”
The study was conducted in the laboratory of Ralph L. Brinster, the Richard King Mellon Professor of Reproductive Physiology at Penn Vet, a renowned scientist of reproductive biology.
Read the full story about this study in Penn Today