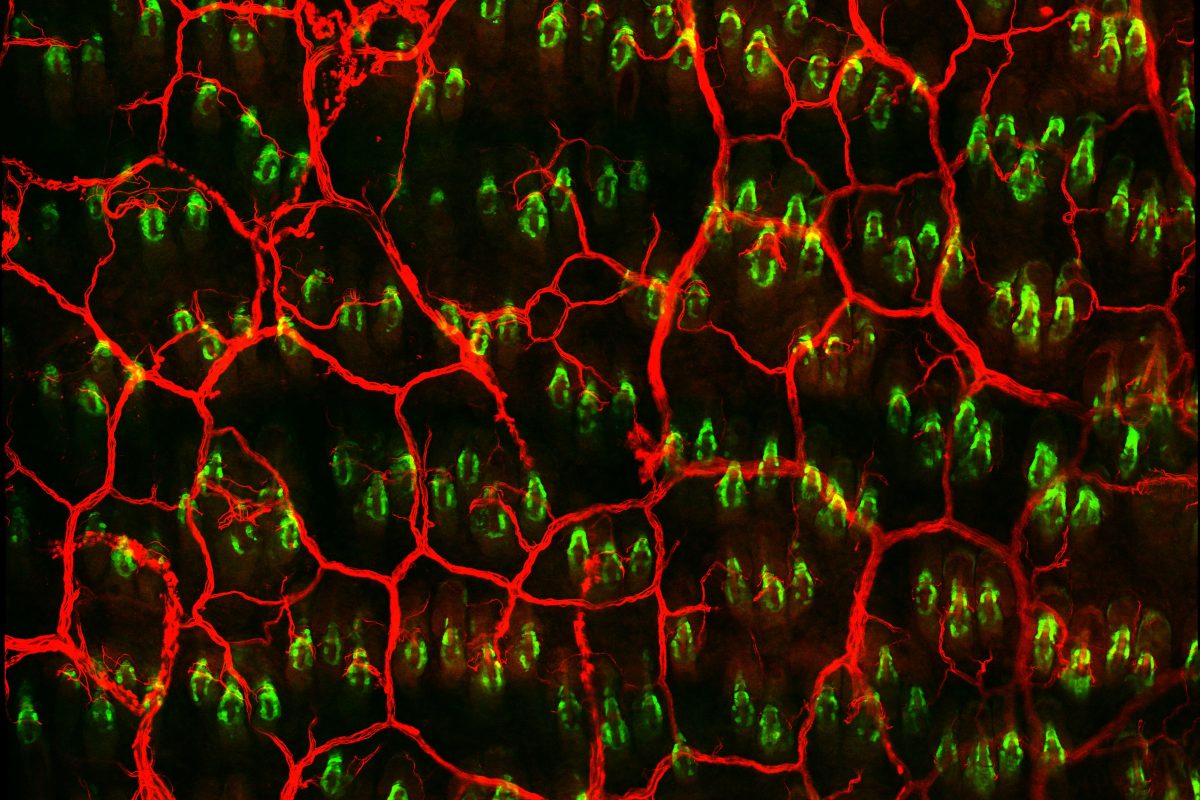
Under normal conditions, a menagerie of separate cell populations work to maintain skin health. However, injury prompts these isolated pools of cells to exit their individual niches and re-epithelialize the epidermis (the surface skin layer). This raises important questions about which cells react first and how the body primes them for rapid injury response.
A study by the Rompolas lab published this week in Cell Stem Cell provides a closer look at how mice maintain these pools of cells and how the cells respond after injury. Led by postdoctoral researcher Sixia Huang, the team used live cell imaging to confirm that that cells expressing Lgr6 serve as first responders that proliferate and initiate epithelial repair following injury. Moreover, the researchers show that targeted destruction of Lgr6+ cells slows the injury response by requiring other stem cells to respond.
So what makes Lgr6+ cells step up first? Consistent with previous data showing that Lgr6 expression by keratinocytes depends on skin innervation, the researchers find that sensory nerves contact the pool of Lgr6+ stem cells and are required for normal cellular dynamics post-injury. This nerve interaction regulates Lgr6+ cell identity and fate by regulating the expression of other genes within the cells, something that is drastically altered if nerves are lost.
Altogether, these results shed light on how the nervous system crosstalks with stem cells in the body. Rather than simply serve as pain receptors, cutaneous nerves prime Lgr6+ cells to function as first responders to injury. These observations may shed light on why patients suffering medical conditions associated with neuropathies, like diabetes, may also suffer from diminished wound healing.
Photo courtesy of Sixia Huang